The Role of Silicone Overmolding in Medical Plastic Device Manufacturing
- Share
- publisher
- siliconeplus
- Issue Time
- Dec 17,2025
Summary
Silicone overmolding enhances medical plastic devices with biocompatibility,design flexibility, and regulatory compliance.
Introduction: Transforming Medical Device Manufacturing with Silicone Overmolding
In the highly regulated and innovation-driven world of medical device manufacturing, the integration of silicone overmolding has emerged as a pivotal technology. This process not only enhances the performance, safety, and durability of medical devices but also opens new avenues for design flexibility and patient comfort. As medical devices become increasingly complex, silicone overmolding offers solutions that meet stringent regulatory standards while delivering superior biocompatibility and functional performance.
Our comprehensive exploration delves into the intricacies of silicone overmolding, its applications in medical plastics, and the significant advantages it provides to manufacturers aiming to elevate their product offerings.
Understanding Silicone Overmolding in Medical Device Manufacturing
What is Silicone Overmolding?
Silicone overmolding is an advanced manufacturing process where a silicone elastomer layer is molded directly onto a pre-existing plastic component. This technique involves injecting liquid silicone into a carefully designed mold that encapsulates the substrate, creating a bonded, seamless interface. The result is a hybrid component that combines the mechanical properties of plastics with the biocompatibility, flexibility, and chemical resistance of silicone.
The Manufacturing Process of Silicone Overmolding
The process typically involves:
Substrate Preparation: Selecting a compatible plastic base, such as polycarbonate, polypropylene, or PEEK, which is pre-formed into the desired shape.
Mold Design: Creating a precision mold that aligns with the substrate's geometry, ensuring optimal coverage and bonding.
Silicone Injection: Injecting medical-grade, biocompatible silicone into the mold cavity under controlled temperature and pressure conditions.
Curing and Demolding: Allowing the silicone to cure, forming a durable elastomeric layer, followed by demolding to reveal the integrated component.
Post-processing: Additional treatments such as surface finishing or sterilization are applied to meet regulatory standards.
Material Compatibility and Selection
The success of silicone overmolding hinges on material compatibility. Medical-grade silicones, such as addition-cure (RTV-2) silicones, are preferred due to their biocompatibility, stability, and ease of sterilization. The underlying plastic substrates are selected based on mechanical strength, transparency, and sterilization compatibility.
Key Advantages of Silicone Overmolding in Medical Devices
Enhanced Biocompatibility and Safety
Silicone's biocompatibility makes it ideal for implantable and wearable devices. Its inert nature minimizes allergic reactions and toxicity, ensuring patient safety. Silicone coatings also act as a barrier against microbial contamination, reducing infection risks.
Superior Mechanical Properties
Flexibility, elasticity, and resilience are hallmark features of silicone elastomers. When overmolded onto rigid plastics, they provide:
Improved grip and tactile feedback in handheld devices.
Shock absorption for delicate instruments.
Enhanced sealing capabilities, crucial for medical connectors and catheters.
Durability and Chemical Resistance
Silicone's resistance to chemicals, temperature fluctuations, and environmental stressors extends the lifespan of medical devices. This resistance ensures consistent performance under various sterilization processes, including autoclaving and gamma irradiation.
Design Flexibility and Customization
Silicone overmolding enables complex geometries, soft-touch surfaces, and color coding for ease of use and identification. This flexibility supports innovative device designs that improve user experience and clinical outcomes.
Improved Sterilization Compatibility
Medical-grade silicones withstand multiple sterilization cycles without degradation, making them suitable for reusable devices. Their non-porous surface minimizes bacterial adherence, facilitating hygiene and infection control.
Applications of Silicone Overmolding in Medical Devices
1. Surgical Instruments and Handheld Devices
Silicone overmolding enhances grip, comfort, and safety in surgical tools such as scalpels, forceps, and endoscopes. The soft-touch silicone grips reduce fatigue and improve precision during procedures.
2. Catheters and Tubing
The flexibility and biocompatibility of silicone make it ideal for catheter coatings, providing sealing, kink resistance, and patient comfort. Overmolding also reinforces connection points, preventing leaks.
Neurostimulators, pacemaker leads, and implantable sensors benefit from silicone's biocompatibility and long-term stability. Overmolding ensures secure encapsulation and reliable insulation.
4. Medical Connectors and Port Systems
Silicone coatings improve sealing performance and user interface, reducing infection risks and enhancing ease of connection/disconnection in IV lines, dialysis systems, and drug delivery devices.
5. Wearable Medical Devices
Devices such as glucose monitors, ECG patches, and infusion pumps gain wearer comfort and durability through silicone overmolding, which provides soft, skin-friendly surfaces.
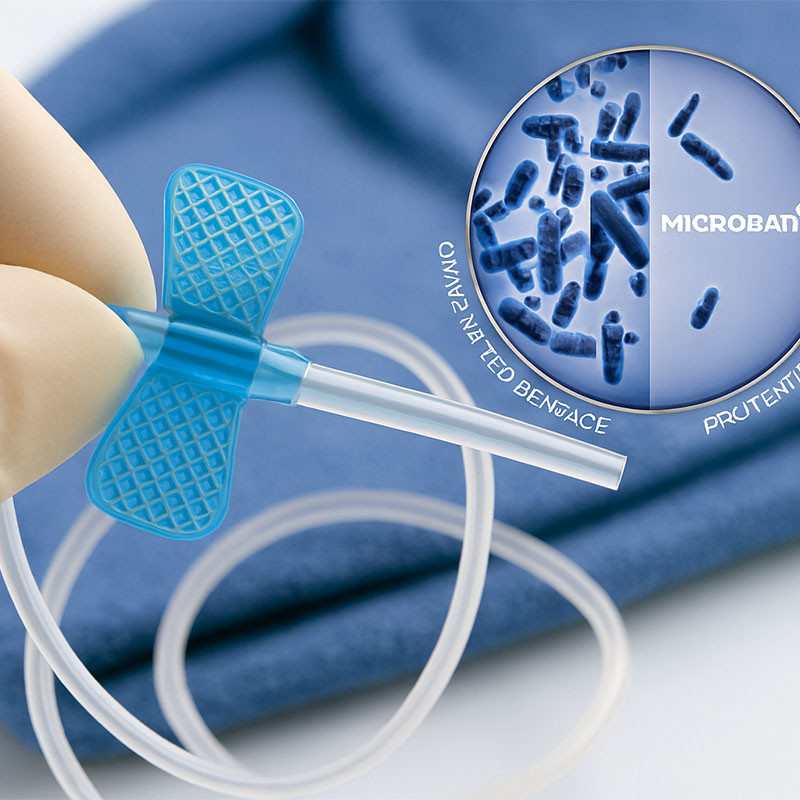
Regulatory and Quality Considerations
Compliance with Medical Standards
Silicone overmolded devices must adhere to ISO 10993 standards for biocompatibility, ensuring non-toxic and non-irritating properties. Additionally, they must meet FDA regulations for medical device safety and efficacy.
Sterilization Compatibility Testing
Rigorous testing under various sterilization methods—autoclaving, ethylene oxide, gamma irradiation—ensures the integrity and
performance of the silicone layer remains intact.
Quality Control and Inspection
Advanced inspection techniques, including ultrasound and infrared imaging, verify bond strength and uniformity of the silicone overmolding, ensuring consistent quality across production batches.
Challenges and Future Trends in Silicone Overmolding for Medical Devices
Addressing Material Bonding Challenges
Achieving a robust bond between silicone and plastics requires optimized surface treatments such as plasma or corona discharge. Ongoing research aims to improve adhesion techniques and material formulations.
Innovations in Biocompatible Silicone Materials
Development of silicone composites with antimicrobial properties and enhanced mechanical properties is a key trend, aiming to further reduce infection risks and extend device lifespan.
Automation and Precision Manufacturing
Advancements in automated overmolding systems and multi-material 3D printing are expected to streamline production, reduce costs, and enable complex device geometries.
Conclusion: Elevating Medical Device Manufacturing with Silicone Overmolding
Silicone overmolding stands at the forefront of medical device innovation, combining biocompatibility, durability, and design flexibility to meet the evolving demands of healthcare. Its ability to enhance device performance, improve patient safety, and support complex geometries makes it an indispensable technology for manufacturers committed to excellence and regulatory compliance.
As the medical industry continues to push the boundaries of device complexity and functionality, the strategic application of silicone overmolding will undoubtedly play a critical role in shaping the future of medical plastics manufacturing. Embracing this technology ensures that medical devices not only meet but exceed clinical expectations, ultimately improving patient outcomes worldwide.
Website:www.siliconeplus.net
Email:sales11@siliconeplus.net.
Phone:13420974883
Wechat:13420974883